Hydroxyapatite is a calcium phosphate compound, its formula is Ca10 (PO4)6(OH)2. It is a part of the raw material, called phosphoric rock. The term hydroxy refers to the anion OH‑. If instead of that anion is replaced with fluoride, the mineral would be called Fluoroapatite Ca10 (PO4)6(F)2. Hydroxyapatite is the main inorganic component of the bones and dental enamel.
Hydroxyapatite predominantly exists in two forms which are nanoHydroxyapatite powder and micron Hydroxyapatite powder. The difference between the two is of size. The size of Hydroxyapatite nanoparticles is below 200 nm whereas micron Hydroxyapatite exists in the range of 45 – 90 micron. The surface area for Hydroxyapatite nanoparticles is around 9.4 m2/g whereas Hydroxyapatite micron powder has a surface area of 120 m2/g.
History and importance of Hydroxyapatite
The use of Hydroxyapatite began after 1950 when after a lot of research, it was found that it can be used in dental surgeries. Since then, its use has continuously increased. It is an important element in the bone tissues of living beings. Its great stability against other calcium phosphate products permits it to withstand physiological conditions, giving the bones their characteristic hardness. Hydroxyapatite fulfills its operation with the aid of collagen, the fibrous protein of connective tissues. Hydroxyapatite consists of Ca2+ ion, but it can also consist of other cations in its structure such as Na+, Mg2+, etc.
Structure of Hydroxyapatite
The below image shows the structure of hydroxyapatite. All spheres occupy the volume of the half of a hexagonal "box", where the other half is identical to the first.
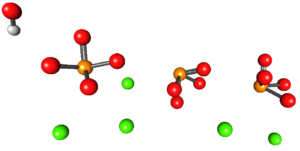
Structure of Hydroxyapatite
In this structure, the green spheres represent Ca2+ cations, while the red spheres represent oxygen atoms, the orange spheres represents the phosphorus atoms, and the white spheres represent OH-.
The phosphate ions in this structure have the defect of not exhibiting a tetrahedral geometry; instead, they look like pyramids with square bases.
It looks like OH- is positioned far from the Ca2+. However, the crystalline unit can repeat itself on the roof of the first, thus presenting the close relationship between both ions. Also, these ions can be substituted by others such as Na+ and F-.
Synthesis of Hydroxyapatite
Hydroxyapatite can be created by the reaction of calcium hydroxide with phosphoric acid:
10Ca(OH)2 + 6H3PO4 => Ca10(PO4)6(OH)2 + 18H2O
Likewise, hydroxyapatite can be produced through the following reaction:
10Ca(NO3)2. 4H2O + 6NH4H2PO4 => Ca10(PO4)6(OH)2 + 20NH4NO3 + 52H2O
The hydroxyapatite nanoparticles can be generated by controlling the rate of precipitation.
Hydroxyapatite crystals
The ions are crushed and grown to form a resistant and rigid bio crystal. This is employed as a biomaterial for bone mineralization. However, it needs collagen, an organic support that acts as a mold for its development. These crystals and their complex formation procedures depend on the bone.
Physical and Chemical properties
- 1.Hydroxyapatite is a white powder that can acquire grayish, green and yellow appearance. As it is a crystalline solid, it has a greater melting point (1100 °C), which indicates strong electrostatic interactions.
- 2.Fluorine (F-) can be substituted by OH- ions in the crystal structure. In this case, it adds resistance to the hydroxyapatite of the dental enamel against the acids. Possibly, this resistance may be due to the insolubility of the formed CaF2, refusing to "leave" the crystal.
- 3.However, in acidic media (as in HCl), it is soluble. This solubility is due to the creation of CaCl2, a highly soluble salt in water. Also, phosphates are protonated (HPO42- and H2PO4-) and interact to a greater extent with water.
- 4.It is denser as compared to water, with a density of 3.05 - 3.15 g/cm3. Moreover, it is practically insoluble in water (0.3 mg/mL), which is because of phosphate ions.
- 5.The solubility of hydroxyapatite in acids is significant in the pathophysiology of caries. The bacteria in the oral cavity secrete lactic acid, a product of the fermentation of glucose, which drops the pH of the dental surface to less than 5 so that hydroxyapatite starts to dissolve.
Applications of nanoHydroxyapatite
Hydroxyapatite nanoparticles have a diversity of applications, which are given below:
- In the surgery of bone tissue, it is employed in the filling of cavities in traumatological, maxillofacial, orthopedic, and dental surgeries.
- The use of hydroxyapatite nanopowder is beneficial in the repairing of enamel and incorporation in toothpaste, as well as mouth rinses.
- It is employed as a coating for orthopedic and dental implants. It is a desensitizing agent used after tooth whitening. It is also employed as a remineralizing agent in toothpaste and in the early diagnosis of caries.
- Due to its resemblance in size, crystallography, and composition with hard human tissue, it is valuable for use in prostheses. Also, nano-hydroxyapatite is bioactive, biocompatible, and natural, as well as not being toxic or inflammatory.
- Titanium and Stainless steel implants are frequently coated with hydroxyapatite nanoparticles to decrease their refutation rate.
- It is a substitute to xenogenic and allogeneic bone grafts. The healing time is less in the presence of hydroxyapatite nanopowder than in its absence.
Applications of micron hydroxyapatite powder
- An alginate-hydroxyapatite complex has been manufactured which is capable of absorbing fluorine through the mechanism of ion exchange.
- Hydroxyapatite micron powder has also been used as a provision for the electrophoresis of nucleic acids. It separates RNA from DNA, as well as DNA from a single strand of two-strand DNA.
- Micron Hydroxyapatite is used in the air filters of motor vehicles to enhance the efficiency of filters in the absorption and decomposition of carbon monoxide (CO). This decreases the environmental pollution.
- Micron Hydroxyapatite is employed as a chromatographic medium for proteins. This presents positive charges (Ca+) and negative charges (PO4-3), so it can interact with electrically charged proteins and permit the separation by the ion exchange process.
Read More About This Product:



Comments
Post a Comment