Clays are used in architecture, industry, and agriculture throughout prehistoric times. It remains one of the essential minerals for industry. Ceramists have used nanoclays without knowing nanoparticles, and they improved the science of clays since antiquity. Furthermore, many applications have been found recently, and resulting indications of nanoclays are reduction of cost and improvement of product quality. Clay minerals can be described as fine-grained hydrous silicates with sheet-like structure, so they are stacked over one another. Different from clays, nanoclays are clay minerals in nanometer range at least one dimension with high aspect ratio.
Figure 1: Nanoclay Morphology. Nanoclay A is pure montmorillonite with Na+ inorganic group, nanoclay B is pure montmorillonite with hydroxyl organic ammonium, and nanoclay C is pure montmorillonite with double alkyl ammonium.

Jiangmiao Yu, Zhibin Ren, Huayang Yu, Duanyi Wang, Shekhovtsova Svetlana, Evgeniy Korolev, Zheming Gao, and Feng Guo, Modification of Asphalt Rubber with Nanoclay towards Enhanced Storage Stability, Materials 2018.
Since clay can be easily obtained, nanoclays can be produced conveniently with relatively low cost, and environment friendly thanks to the contributions to literature on synthesizing and characterization. In addition, surface properties and stability, fabrication of nanoclay-filled nanocomposites, and development of novel materials with nanoclays are also main research fields which resulted tremendous applications. Some of these applications are medicine, pharmacy, cosmetics, catalysis, food packaging, textile industry, environmental protection and remediation.
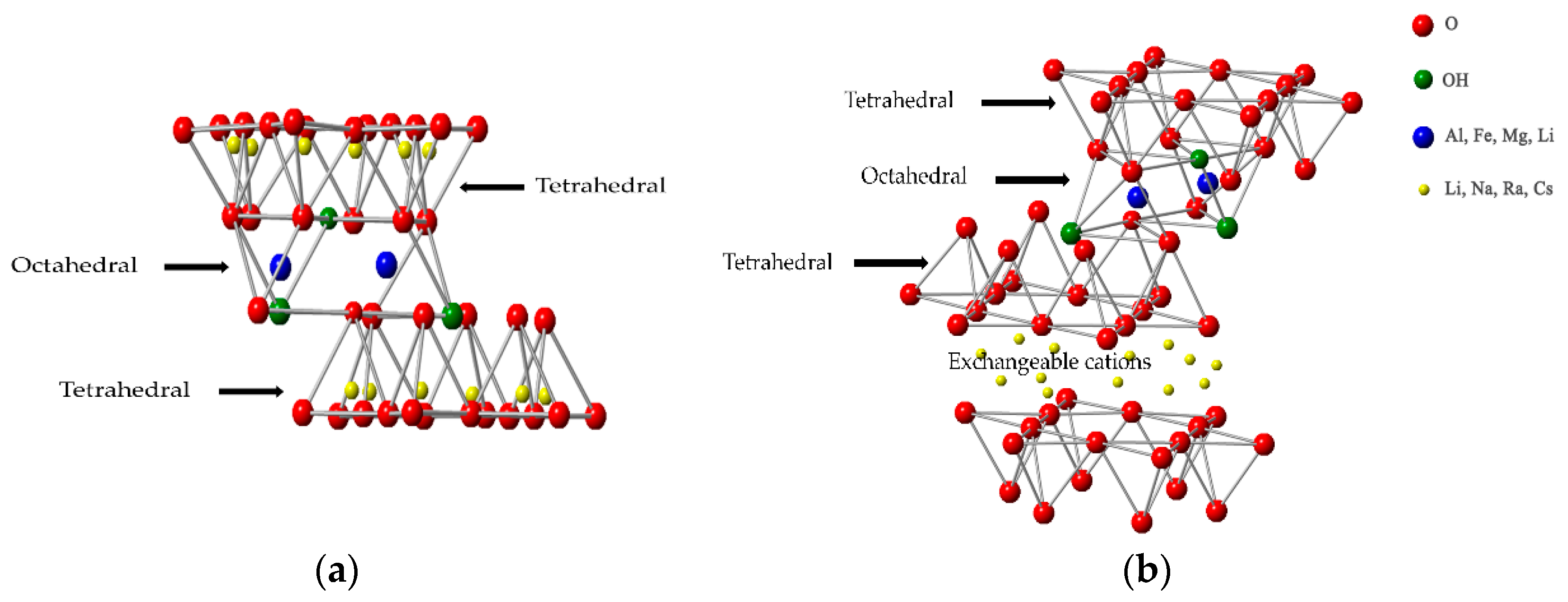
A layer is fundamental structure of nanoclay, each layer is over one another like shelves of library, and the characteristics of nanoclay depend on arrangements of two identical sheets which are tetrahedral and/or octahedral as shown in Figure 2. Clay crystallites are created by layers, and layers are formed by individual sheets. Since there is Van der Waal gaps between each layer, entry into these gaps are possible for water, organic cations or polar organic liquids.
Figure 2: Clay structure of clay minerals (a) Type 1:1; (b) Type 2:1
Feng Guo, Saman Aryana, Yinghui Han, and Yunpeng Jiao, A Review of the Synthesis and Applications of Polymer-Nanoclay Composites, 2018.
Different combinations of tetrahedral and octahedral sheets form three unique sheet arrangements. If one tetrahedral and one octahedral sheets located side by side, It is called Type 1:1, and if one octahedral sheet placed between two tetrahedral sheets, it is called Type 2:1. The last type is Type 2:1:1 when one octahedral sheet bordering another octahedral sheet standing between two tetrahedral sheets per layer. Clay groups for each clay layer type are Rectorite, Kaolinite, Halloysite, Chyrsotile, for type 1:1, Smectite, Vermiculite, Pyrophylite talc, Mica, Brittle Mica, for type 2:1, and Chlorite for type 2:1:1.
It is shown that addition of nanoclays into polymers improve mechanical properties of polymers. In addition to mechanical properties, the permeability of gases such as oxygen, carbon dioxide, organic vapors and moisture can be reduced with using nanoclays in nanocomposites. Furthermore, increase of flammability resistance of nanocomposites and improvement thermal stability have been observed with inclusion of nanoclays. There are many other unique properties for each nanoclay groups leading advancements of numerous applications. As an example research, a group tested the nanoclays in Figure 1 to adjust Asphalt Rubber to improve storage stability.
Easy production of nanoclays availability of clays in nature, make it a magnificent material for industry, technology, and research. One can find various reviews about applications from medicineto environmental protection. Clearly, more applications will be established.
Comments
Post a Comment