Titanium oxide is the one of the top 50 chemicals which are produced in worldwide. It occurs in nature in three forms anatase, rutile and brookite. However, commonly, anatase and rutile forms have been used and studied. Naturally, it has whitish and opaque appearance, and through purification, it becomes whiter. Difference in crystal structure of these two forms as you can see in Figure 1 aroused interest in discrepancies between these forms, and many studies have been conducted.
Figure 1: Crystalline structure of titanium dioxide anatase (a) and rutile (b) Ti and O atoms are represented in white and red respectively.
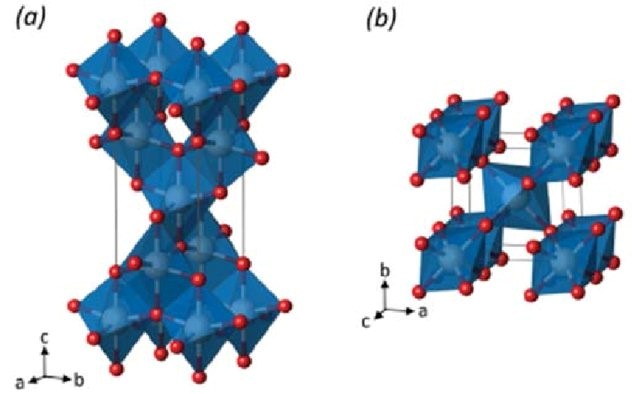
P. Mazzolini, Functional Properties Control of TİO2 for Transparent Electrodes and Photoanodes, 2015
Difference between Characteristics of Rutile and Anatase TiO2 Nanoparticles
In general, scholars studied comparison of photocatalyst and carcinogen characteristics, and production methods between anatase and rutile forms of titanium dioxide nanoparticles. First of all, there is a band gap difference, and anatase has about 3.2 eV band gap, and rutile has about 3.0 eV band gap. Since absorption is inversely proportional to band gap, rutile can absorb more light than anatase. According to photoconductivity measurements, electron-hole pair life time is in anatase is longer than one in rutile, so more charge carriers in anatase participate in surface reactions. In addition, there are researches about toxicology of anatase and rutile titanium dioxide nanoparticles in particular subjects. Results show that anatase is more toxic than rutile. These are the fundamental comparisons, but there are also difference in usage and applications which are both rutile and anatase forms are used.
In a study, N.-G. Park, J. van de Lagemaat, and A. J. Frank working about Comparison of Dye-Sensitized Rutile- and Anatase- Based TiO2 Solar Cells concluded that the short-circuit photocurrent of the rutile-based cell is about 30% lower than anatase-based cell. Another scholars studied on different toxicity of rutile and anatase TiO2 nanoparticles on macrophages: Involvement of difference in affinity to proteins and phospholipids, and the conclusion is that with similar size and zeta potential, rutile and anatase titanium dioxide have different damage effect on organelles in macrophages and different toxicity. The rutile nanoparticles have a high affinity to phospholipids while the anatase nanoparticles have high affinity to proteins.
These differences enhance the range of applications of titanium dioxide nanoparticles. In some applications the difference become an advantage, and both forms are used. Many such applications will be found and developed using the discrepancies.



Comments
Post a Comment