
Image retrieved from:
Silver nanoparticles can be incorporated into products such as photovoltaics, biological and chemical sensors due to its unique optical, electrical and thermal properties. Silver nanoparticles have high electrical conductivity, stability and low sintering temperature so they are used conductive inks, pastes and fillers. Additionally, due to their high novel optical properties, they are preferred as novel material for the molecular diagnostics and photonic devices. Depending on size and shape of the nanoparticles, Silver nanoparticles can absorb and scatter light. Common application area of silver nanoparticles is resulted from their antibacterial natures. Therefore, in many textiles, keyboards, wound dressing and biomedical devices, low level of silver ions can provide a protection against bacteria.
To optimize performance of silver nanoparticles applied into a target, their size, shape, surface and aggregation status are important parameters. Dispersed Silver nanoparticles increase the possibility of non-agglomerated and monodispersed nanoparticles if suitable processing technique is performed for the researches, developments and any other innovative applications. To characterize nanoparticles, different kinds of methods can be used such as transmission electron microscopy (TEM), dynamic light scattering (DLS), Zeta potential measurements and UV/VIS spectral analysis.
When the Silver nanoparticles associate with solvent molecules, the molecules produce a second layer onto surface of the particles providing stabilization and non-aggregated particles. Monodispersed and unaggregated Silver nanoparticles makes the researches understandable in terms of effects on biological system and the environment.
Silver nanoparticle dispersion can be obtained in the solution of water. In water, surface of silver nanoparticles exposed to dissolving by producing silver ions as in the following reaction.
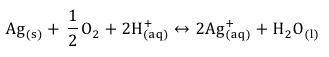
Releasing rate of the Silver ion depends on the size, shape, aggregation state, pH and temperature of the medium. When the size of the particles decrease, the total surface area increases leading to speed the formation of silver ion. Formation of Silver ion is important for the application of Silver nanoparticle in antibacterial purposes. Therefore, dispersion of very small amount of Silver nanoparticles in water are important class of nanomaterials for the wide range of applications.
Products is here: https://nanografi.com/dispersions/ag-in-water-15nm...
https://nanografi.com/dispersions/ag-in-water-2nm-...
Posted by Müberra GÖKTAŞ on January 26, 2017
Comments
Post a Comment